Organo-f-synthesis
Fundamental chemistry of organometallic complexes of heavy elements: study of the Phi bond
If the f-elements, rare earth and actinides, have many applications in various fields, major fundamental questions yet remain to be unraveled. If the buried character of the f-electrons is the origin of important properties in these compounds, this particularly harms the description of a covalent metal-metal bond. More specifically, the interaction between two metallic orbitals of Phi symmetry, the Phi bond, is sorely lacking in the landscape of molecular chemistry.
We build f-elements compounds of high symmetry with original large aromatic ligands of different nature and charge (ligand field). This allows locking the f orbitals in an ideal configuration and facilitates the description of Phi-interactions (metal-ligand pair symmetry). Once the symmetry is locked, the modulation of the 4f- or 5f-metal, including transuranic elements will allow varying the redox state, as well as the relative energy of the f-shell. The redox and physical properties of the compounds with metal-ligand pair symmetry is then engineered with the aim of forming bimetallic species, in which the metal-metal distance is short. This will shed light on the Phi symmetry interactions and favor access to highly original bimetallic species containing a small intermetallic distance, strong magnetic interaction, and unusual reactivity.
The project is an ERC-founded project (2 M€) led by Grégory Nocton and Grégory Danoun (2022-2027).

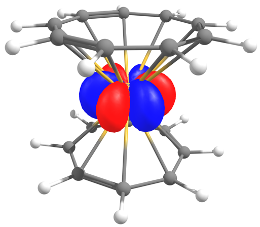
Low-valent uranium complexes for reduced-water electrolysis
The use of depleted uranium in catalysis is an interesting proposition that would enable us to valorize a waste product from the nuclear industry while developing new markets for industrialists with stocks. This is made possible by the specific properties of uranium, which can accommodate numerous degrees of oxidation (+I to +VI), enabling easy redox transformations that vary the number of electrons involved in the transformations. We aim to demonstrate the potential of uranium for these water dissociation reactions. The project is led by Grégory Nocton with Cédric Tard and Grégory Danoun.
Uranyl(VI)-based photo-catalysis
Chemical transformations with a direct C-H activation step appeal to chemists because they allow the functionalization of organic molecules without requiring reactive anchoring functional groups. In this project, we suggest using uranium-based complexes as a promising pioneering family of photoredox catalysts that are not based on precious platinum group metals for C-H activation, including methane.
The project is financed by the ANR (2024-2028) led by Grégory Danoun with Grégory Nocton and Thomas Simler and is performed in collaboration with Cyrille Monnereau and Olivier Maury (ENS Lyon) and Laurent Maron (Toulouse).